Difference Between Ionic and Covalent Bonds
An ionic bond is actually the extreme case of a polar covalent bond the latter resulting from unequal sharing of electrons rather than complete electron transfer. In a true covalent bond the electronegativity values are the same eg H 2 O 3 although in practice the electronegativity values just need to be closeIf the electron is shared equally between the atoms forming a covalent bond then the bond is said to be nonpolar.
Lecture 81 Ionic Vs Covalent 2265011 By Mary Beth Smith Via Slideshare Physical Science High School Science Chemistry Science Classroom
When are reactions reversible.

. For example sodium Na a metal and chloride Cl a nonmetal form an ionic bond to make NaCl. What affects the rate of a reaction. Recognizing Compounds With Ionic Bonds.
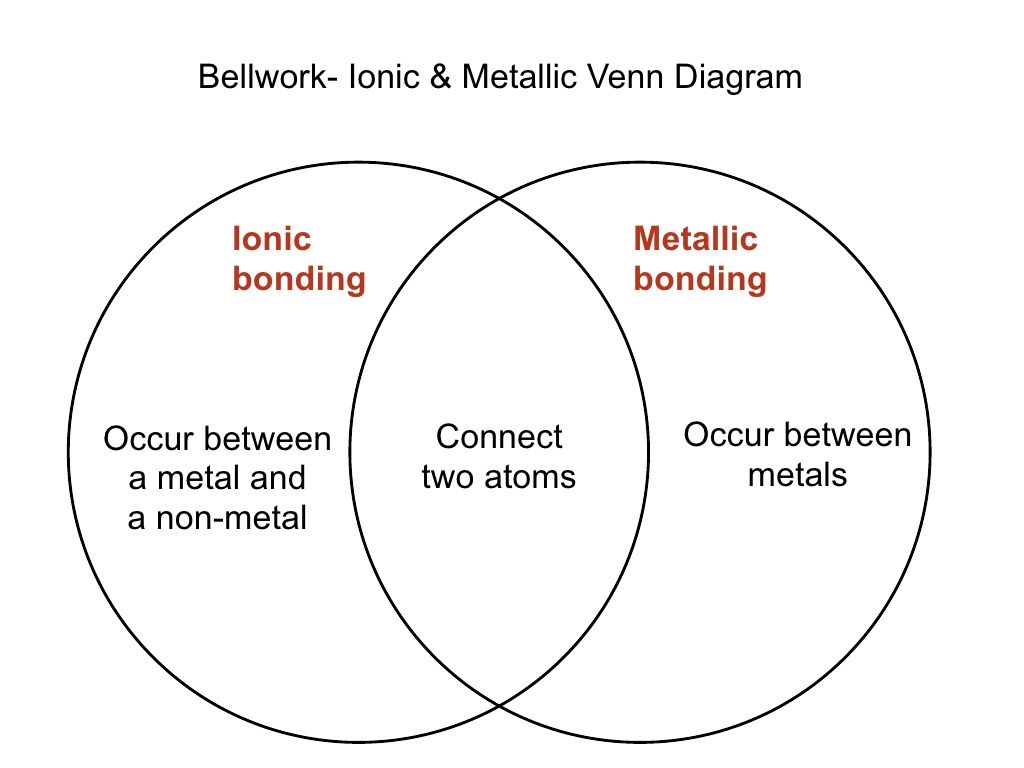
Ionic bonds usually occur between metal and nonmetal ions. The metal atom transfers its electrons to the non-metal atom. Usually covalent bonds formed between the two nonmetals between p-block and p-block and formed when electronegativity difference between atoms exist less than 2.
Ionic bonds typically form when the difference in the electronegativities of the two atoms is great while covalent bonds form when the electronegativities are similar. The difference between an element and a compound is that an element is a substance made of same type of atoms whereas a compound is made of different elements in definite proportionsExamples of elements include iron copper hydrogen and oxygenExamples of compounds include water H 2 O and. In a covalent bond the atoms bond by sharing electrons.
Here is some detail for you. You can recognize ionic compounds because they consist of a metal bonded to a nonmetal. These bonds are different in their properties and structure.
But in ionic bonding cation positive ion and anion negative ion are involved where transfer of elections takes place. According to valence bond theory of which Pauling was a notable proponent this additional stabilization of the heteronuclear bond. The enthalpy of a reaction can be estimated based on the energy input required to break bonds and the energy released when new bonds are formed.
You are even made of atoms. Ionic bonds form between two atoms that have different electronegativity valuesBecause the ability to attract electrons is so different between the atoms its like one atom donates its electron to the other atom in the chemical bond. This two minute animation describes the Octet Rule and explains the difference between ionic and covalent bonds.
The rule is that when the electronegativity difference is greater than 20 the bond is considered ionic. Difference Between Ionic Covalent and Metallic bonds The attractive force which holds together the atoms or group of atoms in a chemical species is known as a chemical bond. Multiple bonds are stronger than single bonds between the same atoms.
Covalent bonds and ionic bonds are types of atomic bonds. Water H 2 O is a covalent compound. For ionic bonds the lattice energy is the energy required to separate one mole of a compound into its gas phase ions.
Difference Between Covalent and Ionic Bonds. While a bond between two ions is called ionic bonds. Design experiments with different reactions concentrations and temperatures.
The definition of the chemical bond as a shared electron pair could be extended to describe the dative bond and the elaboration of Lewis acidbase interactions. Covalent bonds usually occur between nonmetals. Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities and is the primary interaction occurring in ionic compoundsIt is one of the main types of bonding along with covalent bonding and metallic bondingIons are atoms or groups of atoms with an.
Usually an electron is. A covalent bond that has an equal sharing of electrons and the electronegativity difference is zero is called a nonpolar covalent bond. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds.
Because the bond forms between two hydrogens and one oxygen are covalent in nature. If the DEN is between 05 and 16 the bond is considered polar covalent 3. Therefore the metal atom becomes a positively charged cation and the non-metal atom becomes a negatively charged anion.
However in order to make compounds atoms must bond together. If the electronegativity difference usually called DEN is less than 05 then the bond is nonpolar covalent. There are four major types of chemical bonds.
The key difference between covalent and noncovalent bonds is that covalent bonds form when two atoms share their electrons with each other whereas noncovalent bonds form either by completely exchanging electrons between two atoms or by not exchanging any electron. However not all chemical bonds are created equally as you will see in the case of ionic vs. This partial ionic character of covalent bonds increases with the difference in the electronegativities of the two.
Find more free tutorials videos and readin. Explore what makes a reaction happen by colliding atoms and molecules. Covalent bonds ionic bonds hydrogen bonds.
Unlike covalent bonds ionic bonds transfer their valence electrons between atoms. In a covalent bond the atoms are bound by shared electrons. Lattice energy increases for ions.
So lets review the rules. Elements and compounds are pure chemical substances found in nature. Well what is the difference between an ionic and covalent bond.
An ionic bond is a type of chemical bond in which the atoms have different. Explore the difference between ionic and covalent bonds by looking at each different bond and how it forms. A covalent bond is formed between non-metals where sharing of electrons takes place.
For example in water H 2 O each hydrogen H and oxygen O share a pair of. In ionic bonding the electronegativity difference between non-metals and metals exceeds 17. Covalent bonds between identical atoms as in H 2 are nonpolarie electrically uniformwhile those between unlike atoms are polarie one atom is slightly negatively charged and the other is slightly positively charged.
Pauling first proposed the concept of electronegativity in 1932 to explain why the covalent bond between two different atoms AB is stronger than the average of the AA and the BB bonds. Atoms are all around you. The covalent bonds include pairs of electrons by two atoms binding them in a fixed orientation.
Methods of calculation Pauling electronegativity.

Ionic And Covalent Bonding Are Depicted In The Picture Ionic Bonds Is The Attraction Of A Cation To An A Ionic Bonding Teaching Chemistry Chemistry Lessons

College Biochemistry Major Ionic Bond Vs Covalent Bond Covalent Bonding Covalent Bonding Worksheet Ionic Bonding

Difference Between Ionic Covalent And Coordinate Covalent Bond Chemistry Lessons Covalent Bonding Teaching Chemistry

Chemical Bonding Ionic Vs Covalent Bonds Chemical Bond Covalent Bonding Chemistry Projects

Ionic Vs Covalent Vs Metallic Bonds Gcse Chemistry Teaching Chemistry Chemistry Classroom

Difference Between Covalent And Ionic Bonds Chemistry Education Teaching Chemistry Chemistry Classroom
Comments
Post a Comment